Abstract
The impact of donor (D) and recipient (R) CMV serology on transplant outcomes in children with malignant hematological diseases was analyzed in a large registry-based study. Children below 18 years (y) reported to European Society for Blood and Marrow Transplantation (EBMT) who received an unmanipulated transplant from a matched sibling (MSD) or an unrelated donor (UD) (10/10, 9/10 HLA match) with bone marrow (BM) or peripheral blood (PB) between 2007 and 2020 were included. Patients receiving haploidentical grafts, cord blood and ex vivo T-cell depletion were excluded. Impact of D and R CMV serology on overall survival (OS), progression free survival (PFS), relapse incidence (RI) and non-relapse mortality (NRM) was evaluated in 4 categories (D-/R-, D-/R+, D+/R-, D+/R+), within MSD, UD10/10 and UD9/10 groups. Variables used for adjustment were source of cells, age at transplant, female to male transplant, year of transplant, disease risk index, and total body irradiation use.
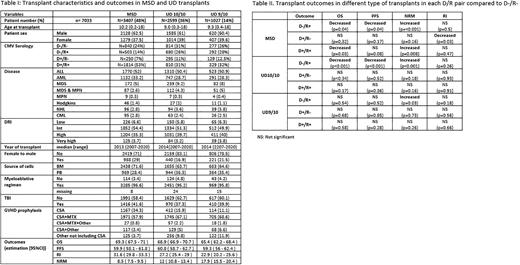
There were 7033 patients with a median follow-up of 3.5 yrs. In total 3407, 2599 and 1027 patients received MSD, UD10/10 and UD9/10 transplants, respectively (Table I). In multivariable Cox models (Table II), MSD with the combinations D+/R+ and D-/R+ had significantly decreased OS (HR, 1.22; p=0.03 and HR, 1.26; p=0.04, respectively) and increased NRM (HR, 1.63; p=0.008 and HR, 2.01; p=<0.001, respectively) compared to D-/R-. The PFS was significantly decreased in D-/R+ (HR, 1.22; p=0.04) compared to D-/R-. For D+/R-, RI was significantly lower (HR 0.71; p= 0.03) without significant increase of NRM (HR 1.5. p=0.15) and non-significantly different PFS (HR 0.83, p=0.17) but RI was not significantly different for D+/R+ and D-/R+ compared to D-/R- (HR 1.06; p=0.47 and HR 1.08; p=0.5, respectively). UD10/10, D-/R+ had a significantly decreased OS (HR, 1.55; p=<0.001), decreased PFS (HR, 1.37; p=<0.001), increased NRM (HR, 2.03; p=<0.001) compared to D-/R- group. In UD9/10, there was no significant difference in OS between groups. Significantly increased NRM (HR, 1.6; p=0.03) was observed in D-/R+ group. Our data show that CMV serology remains to have a substantial impact on survival in both MSD and UD10/10 transplants in children transplanted for malignant hematological diseases. For MSD, the D-/R+ and D+/R+ combinations have decreased OS and increased NRM compared to D-/R- and donor serology is not of significant impact on OS and PFS in both R+ and R- patients. RI was significantly lower in D+/R- in MSD which may be due to the possible "virus versus leukemia” effect as suggested by previous studies. However, this did not translate into improved PFS. For UD10/10, only D-/R+ (but not D+/R+ or D+/R-) have decreased OS and increased NRM compared to D-/R- transplants. This observation is clearly different from previous registry based studies focusing on adults where D+/R- transplants were also reported to have decreased OS compared to D-/R-. Our study reveals that for UD10/10 transplants selection of serocompatible donor is pivotal in R+ patients for transplants in children with malignant hematological diseases. In conclusion, this large retrospective trial shows that CMV serology represents a substantial determinant of survival in both MSD and UD10/10 transplants in children transplanted for a malignant hematological disease. While selecting donor according to CMV serology has no impact on survival for MSD transplants, selection of a serocompatible donor for a seropositive patient significantly improves overall survival in the unrelated donor setting, which represents a clinically impactful finding.
Disclosures
Locatelli:Miltenyi: Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Honoraria; Amgen: Speakers Bureau; SOBI: Speakers Bureau; BlueBird bio: Speakers Bureau; Neovii: Speakers Bureau; Medac: Speakers Bureau. Biondi:Amgen: Honoraria; Novartis: Honoraria; Bluebird: Other: Advisory Board; Incyte: Consultancy, Other: Advisory Board. Fagioli:TAKEDA: Membership on an entity's Board of Directors or advisory committees, Research Funding; GENZYME SANOFI: Membership on an entity's Board of Directors or advisory committees, Research Funding; CLINIGEN: Membership on an entity's Board of Directors or advisory committees, Research Funding; JAZZ PHARMA: Membership on an entity's Board of Directors or advisory committees, Research Funding; PFIZER: Membership on an entity's Board of Directors or advisory committees, Research Funding; NOVARTIS: Membership on an entity's Board of Directors or advisory committees, Research Funding; AMGEN: Membership on an entity's Board of Directors or advisory committees, Research Funding. Meisel:Bellicum: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Miltenyi Biotech: Research Funding; Neovii: Research Funding; Gilead/KITE: Research Funding; CRISPR Therapeutics/Vertex: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Research Funding; Celgene BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; MEDAC: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.